Page 1 of 2
Sulfur, oil and gas by-product information webpage

Posted:
Wed 06 Oct 2004, 02:03:58by Keith_McClary
In this discussion:
http://peakoil.com/fortopic1917.html
"Saudi extra barrels wrong kind of crude!"
big_rc asked:
"what do the refineries do with the sulphur they remove from the oil?"
so I made this web page:
http://www.cuug.ab.ca/kmcclary/sulfur/

Posted:
Wed 06 Oct 2004, 03:08:23by rowante
Nice work Keith!
More from
here.
Sulfuric acid is prepared industrially by the reaction of water with sulfur trioxide, which in turn is made by chemical combination of sulfur dioxide and oxygen either by the contact process or the chamber process. The lead chamber process is used to produce much of the acid used to make fertilizers. It produces a relatively dilute acid (62% - 78%). The contact process produces a more concentrated acid but requires purer raw materials and the use of expensive catalysts. Some sulfuric acid is also made from ferrous sulfate waste solutions from pickling iron and steel and from waste acid sludge from oil refineries.
The uses of sulfuric acid are so varied that the volume of its production provides an approximate index of general industrial activity. Its main use is in phosphate fertilizer production, both superphosphate of lime and ammonium sulfate. It is widely also used to manufacture chemicals, e.g., in making hydrochloric acid, nitric acid, sulfate salts, synthetic detergents, dyes and pigments, explosives, drugs, other acids, parchment paper, glue and wood preservatives. It is used in the purification of petroleum to wash impurities out of gasoline and other refinery products.Sulfuric acid is used in processing metals, e.g., in pickling (cleaning) of metal, electroplating baths, nonferrous metallurgy. Rayon is made with sulfuric acid. In one of its most familiar applications, it serves as the electrolyte in the lead-acid storage battery commonly used in motor vehicles (acid for this use, containing about 33% H2SO4 and with specific gravity about 1.25, is often called battery acid).
And
here.The largest sources of elemental sulfur are petroleum refining and natural gas processing at numerous facilities throughout the United States. Elemental sulfur is mined at a few locations worldwide. Smaller quantities of sulfur are recovered as sulfuric acid at nonferrous metal smelters, and minor amounts are recovered at coking operations. Between 11 and 12 Mt/yr of domestic sulfur in all forms are produced. The United States imports about 3 Mt/yr of sulfur as elemental sulfur and sulfuric acid. Exports total less than 1 Mt/yr. The majority of U.S. imports come from Canada, the largest sulfur exporter in the world. Annual apparent consumption is almost 14 Mt.

Posted:
Wed 06 Oct 2004, 08:28:19by big_rc
This website never ceases to amaze me. I learn something new almost everytime I log on here.
Thanks for the info !!
Re: sulfur, oil and gas by-product

Posted:
Wed 06 Oct 2004, 16:16:43by Rembrandt
great webpage!
Very informative clear and it gives you the idea within half a minute
my compliments
Re: sulfur, oil and gas by-product

Posted:
Thu 01 Dec 2005, 01:53:12by Keith_McClary
Russian Scientist Suggests Burning Sulfur in Stratosphere to Fight Global Warming
Just to put this in perspective, the amount of sulfur he is proposing to burn is about half of this stockpile:
http://www.cuug.ab.ca/kmcclary/sulfur/
BTW, the stockpile has been almost all melted down and shipped away in the year since I made the webpage.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Mon 13 Mar 2006, 07:15:54by grabby
Lets see, am I hearing this right?
We removed all the sulfer from our oil to save the air,
Now we have to burn it in the air to save the planet?
Who screwed this one up?
But by Removing all the sulfer from the oil processing refineries to SAVE the ecology, we have then caused the global warming?
Reminds me of ants I saw when I was little, a couple thousand were stacking up sand in one area and another couple thousand were unstacking it. the unstackers were winning.
But reality is probably the truth:
Russia probably has high sulfer oil, and it is expensive to extract it, so the Russians paid a scientist to come up with a reason for leaving sulfer in the oil and this is their explanation, thus raising Russias high sulfur oil value with the stroke of a pen.
Hey I could become a politician.
Except I would have to overcome my problem with honesty.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Mon 13 Mar 2006, 09:16:26by Doly
Sulfur in other fuels causes acid rain. I guess it's only sulphur in the stratosphere that is "safe". Of course, it may not be so safe after all. In the end, it all depends on whether the remedy is worse or better than the disease.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Fri 17 Mar 2006, 01:27:05by aflatoxin
Much of our natural gas is loaded with hydrogen sulfide. Evil, deadly gas, second only to HCN in toxicity.
The old way was to use a process to remove it from the gas using an amine process similar to that used to remove CO2 from gas. THe H2S was then burned in a flare or candlestick incinerator. (H2S is very flamable) this resulted in a lot of SO2 emissions. (not to mention the H2S that was not burned.
More modern methods used something called a Sulfur Recovery Unit (SRU) Sulfur had economic value for use for fertilizer and such. In the Klaus process, 2/3 of the H2S was burned in the air to make SO2, then reacted with the other 1/3 of the H2S on a catalyst to make elemental sulfur and water. Pretty slick. The tail gas from this process was run through the flare or candlestick as above.
Since Sulfur is bad for our planet, efforts have been underway to reduce the amount released into the air. The above two process have been limited by both environmental regulations and a glut in the global sulfur market. It's hard to get rid of sulfur nowadays.
So, the more modern method is to strip the H2S out of the gas, then reinject it into the ground. The H2S is 40% or more, balance CO2, then run down a wellbore, and gone.
One of the more interesting places I've been liquifies the H2S prior to reinjection. The same place scavenges the waste gas from the processing of NG and runs a pretty big power plant of the waste gas.
On another note, most of the CO2 stripped from gas (and it is a LOT of CO2) is blown into the sky as a waste product. The above mentioned plant shipped their waste CO2 hundreds of miles (pipeline) to reinject into oil-bearing formations to improve production. I'm not going to name them by name, but they don't (publicly) believe in PO. Although they are a very large and very evil corporation, they eat every part of the buffalo.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Fri 17 Mar 2006, 01:44:22by aflatoxin
Another place I worked at took H2S from a nearby NG processing plant, then burned it to make SO2>SO3>(H2SO4) (Anhydrous sulfuric acid).
Pretty scary stuff. They took this and put it into a reactor with common salt (NaCl) to make HCl gas> hydrochloric acid. This was used to pour down oil wells to dissolve limestone and improve production.
This was, and is, hands down, the most dangerous place I've ever been.
I only got gassed by the HCl when the line feeding the RV to the tails scrubber blew, but I barely made it out before I passed out. The H2SO4 contaminates in the gas cloud dissolved my tee-shirt. Were it not for my air-pac, I probably would have died. Rumor has it that people came off the tower only wearing their zipper.
(BTW, this plant was in the US, it was closed in 1999 when low oil prices killed the demand for HCl)
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 09 Apr 2006, 02:02:34by Keith_McClary
aflatoxin wrote: Although they are a very large and very evil corporation, they eat every part of the buffalo.
You mean Kerr-Mc Evil?
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 09 Apr 2006, 05:33:42by aflatoxin
Close, but no.
XOM
Had some good times with kermac too.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 02 Jul 2006, 21:46:26by Aimrehtopyh
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 16 Mar 2008, 02:52:47by keenwoood
Keith_McClary, you can add your webpage here
Wood products woodworking industry directory
I think it is very informative!
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sat 18 Oct 2008, 14:32:59by PradeepIV
From the
Green Chemicals Blog
"..according to researchers from the US Department of Energy's Argonne National Laboratory and Ontario, Canada-based Kingston Process Metallurgy Inc. (KPM), you can also extract pure hydrogen from the hydrogen sulfide that naturally contaminates unrefined oil including oil sands."
IMO, this is significant, because :
1. Sulfur production (and conversely H2 production) from oil-sands projects could approach tens/hundreds of tonnes of sulfur/d.
2. The Claus process currently used to produce sulfur from hydrogen sulfide (H2S) uses air, resulting in the hydrogen being converted to water. This H2 can be recycled back to the processing steps in the process, decreasing the need for converting natural gas to supply the hydrogen.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Thu 18 Dec 2008, 02:11:42by sparky
.
one of the use of sulfuric acid is when concentrated at 98% to dry stuff , at such high concentration it is not corrosive and can be kept in normal steel tanks , but it is deadly , fear is the proper attitude when handling this tiger
Sulfur is not bad for the planet ,
plants use it to grow and there is no problem there ,
the sulfur in the oil came from the vegetation
you can treat your garden with it , plants love it
the problem is local excess and the form ,
usually sulfur dioxyde is OK but if too concentrated instead of making the rain lightly acid it become concentrated acid ,
morning mist in the valleys is even worst , rain doesn't wash it down
the mist droplets have been recorded at PH 1 , that's a killer
a pity because the production of SO2 is probably the biggest climate modifying gas humans produce and yes
...... it's a cooling effect
I've worked with sulfuric and sulfurous acid SO2 ,SO3 and H2S , this last one is an integral bastard
It make steel brittle and develop cracks , brass is destroyed too
so it tend to leak
It's explosive , quite violently too and will maintain a fire with toxic smoke
It stink , when it doesn't either there is none or there is so much your nose is desensitised and your going to die !
Re: sulfur, oil and gas by-product

Posted:
Wed 20 May 2009, 02:22:42by ronzzkee
yeah good luck guys , i know...they are getting better
_________________
Vinyl Banners |
My Sports
Re: Sulfur, oil and gas by-product information webpage

Posted:
Thu 21 May 2009, 03:45:28by sparky
.
Sound like the Chineses are stockpiling big time ,
probably on all the essential raw materials too
.
Re: Sulfur, oil and gas by-product information webpage

Posted:
Fri 21 Aug 2009, 23:27:05by rc2429
Great web page+content and nice info
Thanks
RC
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 14 May 2017, 19:38:25by Subjectivist
Shoutout to the oil and gas industry folks around here. I am researching sulfur and specifically sulfur pipelines. I am having trouble finding information on the corrosion resistant pipelines used to ship the liquified sulfur at high temperature.
How hard is it to manufacture pipes that resist corrosion from high temperature pure sulfur?
Sulfur goes through an interesting viscosity change as it melts and heats up. It starts out fairly low viscosity, then when it hits a critical temperature the viscosity shoots up very high. Then as the temperature continues to rise it eventually gets hot enough that it once again has low viscosity, then a dozen degrees hotter it boils into vapor.
Because of this viscosity swing pipelines keep the temperature in the first low viscosity range with heating systems, usually a hot water jacket around the sulfur pipe.
http://www.cheresources.com/claus1.gif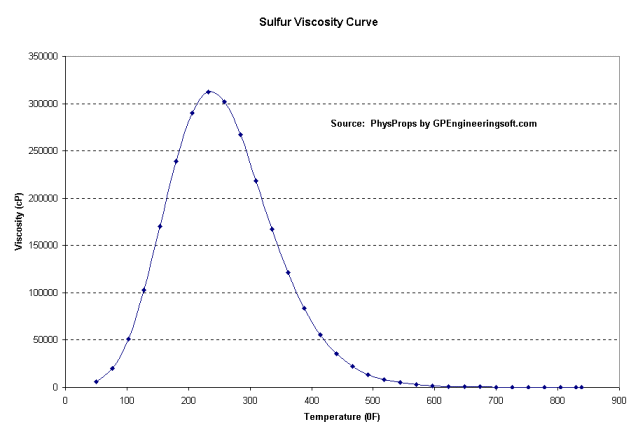
Re: Sulfur, oil and gas by-product information webpage

Posted:
Sun 14 May 2017, 22:01:41by ROCKMAN
Sub - Here's something for you to chew on. From:
http://www.ogj.com/articles/print/volum ... years.htmlWorld's longest sulfur pipeline operating smoothly after 3 yearsMore than 3 years of trouble-free operation on the world's longest liquid-sulfur pipelines have affirmed the attention to detail and the early engineering effort that solved the problems of transporting liquid sulfur in a buried pipeline at temperatures greater than 120° C.
Careful engineering and project planning led to completion on time and under budget of the 24.6 mi long line. Sulfur is extracted from sour gas at Shell Canada Ltd.'s Caroline gas plant, Alta. It is carried cross-country as a liquid to the nearest railhead to the south.
The pipeline is built from two coaxial pipes. The inner pipe carries liquid sulfur, while the annular space between the inner and outer pipes carries circulating hot water under pressure. A return line completes the loop for the circulation of hot water.
